Medical device registration in GCC
Different countries can have unique ways to classify various products, and they have different regulations for marketing and selling those products. In this case study, we explore a situation where a medical device was classified differently in the country of origin than the country it was being registered in, which caused issues regarding certifications and government approvals.
The country where our client needed their product registered had classified the product as a medical device. However, it was classified as a pharmaceutical product in the country of origin. On further discovery, it turned out that the classification was somewhat ambiguous. The challenge here was to find the optimal solution based on several factors.
- Published in Case Studies
Regulatory Inroads by GCC Health Authorities in Times of COVID19?
- Published in Articles
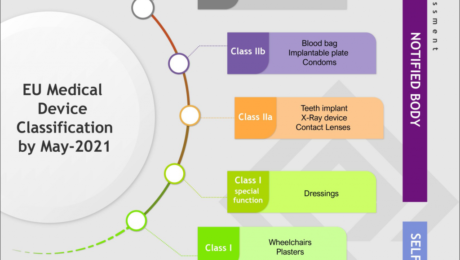
How the EU Medical Device Regulation will affect the GCC?
- Published in Articles
Medical Devices Regulations in Oman – October 2020?
- Published in Articles
How to progress in your Regulatory affairs career?
- Published in Articles
The aesthetic cosmetic industry is witnessing an upsurge in the Middle Eastern region.?
- Published in Articles
Pharma Digitization: Why to get implemented and what does it hold for the Pharmaceutical Industry??
- Published in Articles
Biosimilar Regulations in Egypt
Title: Biosimilar Regulations in Egypt
Date: 22nd September 2021
Time: 1:00 PM (UAE Time)
In the fifth episode, we will discuss the Biosimilar Regulations in Egypt.
Episode 05 Guest:
Asmaa Fouad Ismail
General Director of Biological Products General Directorate and Emergency Committee Member
- B Sc. of pharmacy, Cairo university, class 2002.
- Currently working as general director of biological products general directorate and emergency committee member.
- Former head of Biologicals Registration at NORCB.
- Worked in Registration of biological products since 2003 till 2021.
- Participated in establishment of the latest version of the Biosimilar Registration guideline, emergency use approvals guideline and team member in registration group of the National project to produce plasma for fractionation.
- A member in WHO global review team for Covid -19 vaccines.
- Represented the Egyptian health authority as a speaker during the Middle East regulatory conference.
To view episode 5, please click the links below:


- Published in Previous Episodes